Where are you?
To give you the most accurate information about our products and what we can do for you, we need to direct you to the right site.
To give you the most accurate information about our products and what we can do for you, we need to direct you to the right site.
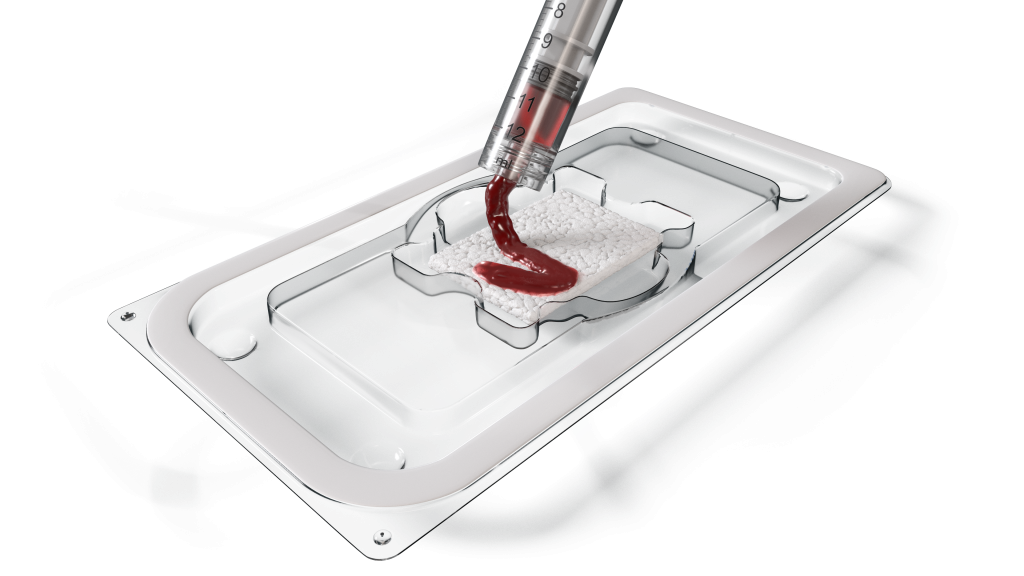
Move the needle in spinal fusion:
with our open fibrillar matrix
MagnetOsTM Flex Matrix provides our pro-healing NeedleGripTM surface technology with immediate access to cancellous bone: for a more predictable fusion.
MagnetOs Flex Matrix is an open matrix bone graft with a unique fibrillar – and flexible – structure that optimizes the effect of our established NeedleGrip surface technology. By exposing the NeedleGrip surface to the surrounding biological environment, MagnetOs grows bone even in soft tissue* without added cells or growth factors.1-3†‡
Furthermore, this product gives you greater efficiency in the operating room, thanks to its exceptional wicking properties. In fact, MagnetOs Flex Matrix absorbs up to ten times as much BMA as a leading competitor’s bone graft.4
MagnetOs Flex Matrix is also extremely convenient to use with excellent granule retention. It stays strong yet flexible even when wet: so you can tear it, mold it, and fold it to fill bony spaces any way you need, including in the intervertebral disc space or posterolateral spine.4,5,§
Flexible
MagnetOs Flex Matrix features an open fibrillar – and more flexible – design comparable to native collagen, rather than the cross-linked, sheet-like morphology often seen in other bone grafts.
Practical & versatile
Because it features the fibrillar structure of natural collagen, MagnetOs Flex Matrix retains its strength and shape while remaining flexible. It’s convenient to handle. It isn’t brittle when dry; nor does it break up and become soggy when wet.4
All of which makes this product incredibly versatile. Simply tear it, mold it, and fold it any way you need to fill irregularly shaped bone defects, gaps and voids.
High wickability & granule-volume percentage
MagnetOs Flex Matrix absorbs up to ten times as much BMA as a leading competitor’s bone graft – providing more cells to support bone growth. It also carries and retains a higher volume percentage of granules.4 Wet cohesivity is designed into this bone graft so that granules don’t shed easily, even when hydrated and can be used in the posterolateral spine and intervertebral disc space.5,§

Propagation in action
Histology revealed significant graft resorption complemented by abundant bone tissue and continuous bony bridging between transverse processes, resulting in spinal fusion in all implants treated with MagnetOs Flex Matrix.†
Conversely, continuous bone bridging was not observed and therefore not considered fused for any of the implants treated with i-FACTOR® Putty.4†¶
Clinical Storyboard

Dr. Kathryn McCarthy is a fellowship-trained orthopedic surgeon at Norton Leatherman Spine Center in Louisville, Kentucky. She has 12 years of practice, focusing on adult degenerative pathology and deformity cases throughout the spine.
In the interactive Clinical Storyboard below, one of Dr. McCarthy’s patients is followed before and after a fusion using MagnetOs Flex Matrix at 6 weeks, 12 weeks and 9 months.
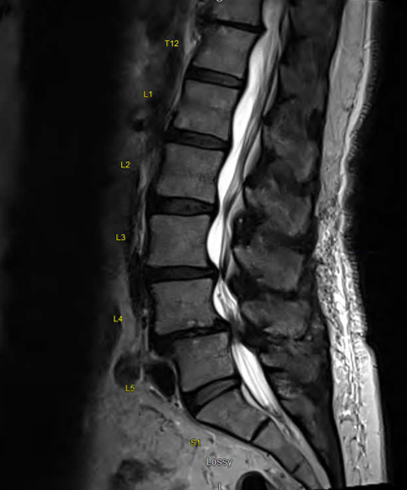
Lateral X-ray reveals disc space collapse at L4-5 and some degree of disc space narrowing L3-4
AP X-ray reveals advanced facet arthropathy, most notable on the right at L4-5; asymmetry in disc height was evident at L3-4
MRI shows stenosis both in foraminal and central space at both levels and associated fluid within facets, notably at L4-5
Proceeded with 2-level posterior fusion with wide decompression bilaterally and interbody grafting for anterior column restoration

Maintained alignment, restoration of disc height at L3-4 and L4-5
Grafting material visible posterior to the cage and posterior gutter
6 week postoperative clinical observations: ODI 18, VAS back 3, leg 0
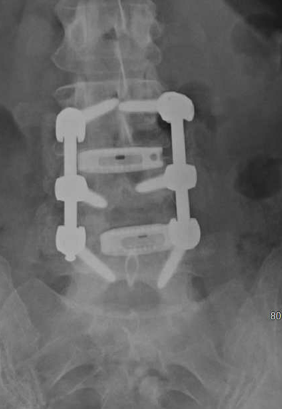
MagnetOs appeared less granular as fusion mass matures
Maintained lordosis and disc space height
Maturation of graft material in posterolateral gutters and posterior to cage

Mature fusion mass in posterior gutters from L5 to L3
Maturation of graft material posterior to cage
MagnetOs Flex Matrix in the OR
Product code Volume Dimensions (mm) Granule size (mm)
703-056-US Small ≥ 28 L x 28 W x 3 H 0.25-1
703-057-US Medium ≥ 48 L x 35 W x 3 H 0.25-1
703-058-US Large ≥ 96 L x 35 W x 3 H 0.25-1
703-059-US Extra Large ≥ 96 L x 35 W x 4.5 H 0.25-1
Want to learn more about MagnetOs Flex Matrix?
Book your own appointment with one of our scientific experts.
1. Duan, et al. eCM. 2019;37:60-73.
2. Van Dijk, et al. eCM. 2021;41:756-73.
3. Van Dijk, et al. J Immunol Regen Med. 2023;19:100070.
4. Data on file.
5. Please refer to the Instructions for Use (IFU) MagnetOs Flex Matrix (US) for a full list of indications, contraindications, precautions and warnings.
* In large animal models.
† Results from in vitro or in vivo laboratory testing may not be predictive of clinical experience in humans. For important safety and intended use information please visit kurosbio.com/eifu.
‡ MagnetOs is not cleared by the FDA or TGA as an osteoinductive bone graft.
§ When used in intervertebral body fusion procedures, MagnetOs must be used with an intervertebral body fusion device cleared by FDA for use with a bone void filler.
¶ i-FACTOR Putty is indicated at one level from C3-C4 to C6-C7. See i-FACTOR IFU for full details.
PROMO/MAG/US/029-23/R01